I was switching out the bobbin on my sewing machine, and looking for a white cotton one to match my new thread. The problem was that I have two white threads: cotton and polyester. Which one was in the bobbin? I couldn't tell just by looking at it.
So I whipped out the matchbox and performed the tried-and-true burn test. Anyone who's familiar with textiles knows that natural fibers like cotton and wool burn, but synthetic ones melt into a little blob. The thread on my bobbin burned, proving it cotton, and I went merrily on my way.
But then later I found myself asking: why do natural fibers burn but synthetic ones don't? Surely there must be a chemical reason. Digging around on Wikipedia didn't reveal any easy answers.
First of all, let's lay down some definitions: natural fibers come from plant, animal or mineral sources. Common examples of plant fibers are cotton, linen (via flax), and hemp. Animal fibers come from a variety of mammals including sheep, goats, alpaca, and rabbits for wool and fur. Silks are also animal fibers, and come from animals like silkworms and clams. The most well known mineral fiber is asbestos; mineral fibers aren't used for textiles.
Man-made fibers break down into roughly two categories: regenerated and synthetic. You'll see the word artificial used as well, but it seems to have an ambiguous meaning. Anyway, synthetic fibers come from chemical reactions. All the sources I've read talk about extrusion, but I think this is best explained with an example.
When I took organic chemistry, we synthesized nylon. The very basic idea is that you have two chemicals, one floating on top of the other. In between, a reaction creates nylon. If you pull the nylon out, more nylon will be created, giving us a nylon thread. Here's a video example. I imagine fancy machinery does this at a smaller scale to create the stuff we use regularly.
My understanding is that regenerated fiber is created with basically the same process, except one of the chemicals is a cellulose pulp from a natural source, like wood, bamboo, or seaweed. Rayon, an "artificial silk," is one of the most common fibers of this kind, and is made from wood.
Now let's get back to the burn test. Natural fibers burn in various ways, synthetic fibers melt, and regenerated fibers burn a little and melt a little—not terribly surprising. But why does the distinction exist in the first place?
Cotton, linen, and other plant fibers are made mostly of cellulose, which has the ring-like structure shown below. When cellulose burns, it takes in oxygen and puts out carbon dioxide and water. As a balanced chemical equation: C6H10O5 + 6O2 --heat--> 6CO2 + 5H2O. The output are gasses, so they just float away. Ash that remains from burning anything is mostly made up of metal oxides, so I'm guessing it comes from the non-cellulose bits.
Animal fibers are made of more complicated proteins made from carbon, hydrogen, oxygen, nitrogen and sulfur and are called polyamides. I'm imagining that they have similar (if more complex) chemical reactions that result in carbon dioxide, water, and other gases, as well as ash. Nitrogen gas (N2) is common in the atmosphere, and Ammonia, or NH3 might explain the odor associated with burning animal fibers. Hydrogen sulfide (H2S) also has a bad smell.
Nylon is also a polyamide. It contains a fair amount of nitrogen, and burning it produces the dangerous hydrogen cyanide. What I really want to understand is the following line (from the nylon Wikipedia page) on the chemical structure of nylon: "The direction of the amide bond reverses between each monomer, unlike natural polyamide proteins which have overall directionality: C terminal → N terminal." I have only a vague idea what it means, and no idea if it's even relevant.
Really, I just need to corner a chemist and harass them until they give me some straight answers. Or find a resource on the chemical reactions involved in burning wool, nylon, and rayon. So far, I've no luck.
So I whipped out the matchbox and performed the tried-and-true burn test. Anyone who's familiar with textiles knows that natural fibers like cotton and wool burn, but synthetic ones melt into a little blob. The thread on my bobbin burned, proving it cotton, and I went merrily on my way.
But then later I found myself asking: why do natural fibers burn but synthetic ones don't? Surely there must be a chemical reason. Digging around on Wikipedia didn't reveal any easy answers.
First of all, let's lay down some definitions: natural fibers come from plant, animal or mineral sources. Common examples of plant fibers are cotton, linen (via flax), and hemp. Animal fibers come from a variety of mammals including sheep, goats, alpaca, and rabbits for wool and fur. Silks are also animal fibers, and come from animals like silkworms and clams. The most well known mineral fiber is asbestos; mineral fibers aren't used for textiles.
Man-made fibers break down into roughly two categories: regenerated and synthetic. You'll see the word artificial used as well, but it seems to have an ambiguous meaning. Anyway, synthetic fibers come from chemical reactions. All the sources I've read talk about extrusion, but I think this is best explained with an example.
When I took organic chemistry, we synthesized nylon. The very basic idea is that you have two chemicals, one floating on top of the other. In between, a reaction creates nylon. If you pull the nylon out, more nylon will be created, giving us a nylon thread. Here's a video example. I imagine fancy machinery does this at a smaller scale to create the stuff we use regularly.
My understanding is that regenerated fiber is created with basically the same process, except one of the chemicals is a cellulose pulp from a natural source, like wood, bamboo, or seaweed. Rayon, an "artificial silk," is one of the most common fibers of this kind, and is made from wood.
Now let's get back to the burn test. Natural fibers burn in various ways, synthetic fibers melt, and regenerated fibers burn a little and melt a little—not terribly surprising. But why does the distinction exist in the first place?
Cotton, linen, and other plant fibers are made mostly of cellulose, which has the ring-like structure shown below. When cellulose burns, it takes in oxygen and puts out carbon dioxide and water. As a balanced chemical equation: C6H10O5 + 6O2 --heat--> 6CO2 + 5H2O. The output are gasses, so they just float away. Ash that remains from burning anything is mostly made up of metal oxides, so I'm guessing it comes from the non-cellulose bits.
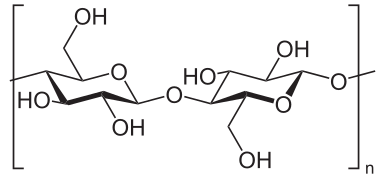 |
| Cellulose, via Wikipedia. |
Animal fibers are made of more complicated proteins made from carbon, hydrogen, oxygen, nitrogen and sulfur and are called polyamides. I'm imagining that they have similar (if more complex) chemical reactions that result in carbon dioxide, water, and other gases, as well as ash. Nitrogen gas (N2) is common in the atmosphere, and Ammonia, or NH3 might explain the odor associated with burning animal fibers. Hydrogen sulfide (H2S) also has a bad smell.
Nylon is also a polyamide. It contains a fair amount of nitrogen, and burning it produces the dangerous hydrogen cyanide. What I really want to understand is the following line (from the nylon Wikipedia page) on the chemical structure of nylon: "The direction of the amide bond reverses between each monomer, unlike natural polyamide proteins which have overall directionality: C terminal → N terminal." I have only a vague idea what it means, and no idea if it's even relevant.
Really, I just need to corner a chemist and harass them until they give me some straight answers. Or find a resource on the chemical reactions involved in burning wool, nylon, and rayon. So far, I've no luck.

5 comments:
Looking at this illustration will help tremendously, I think: http://pslc.ws/macrog/lab/images/fiber02.gif
As you quoted, the direction of the amide bond is alternating along the length of the polymer. This is important because it allows polymers beside each other to connect through hydrogen bonds, forming a very rigid, structured, but long fiber.
The heat from the match apparently broke these alternating H-bonds, turning the whole pattern into an unstructured "blob" of its constituent, now unconnected polymers.
I'm just finishing my associate's degree, but am going to transfer and major in chemistry soon, so don't take my word for it (yet!).
I think that is helpful: it makes sense that the H-bonds would break down before anything else. So the other stuff does burn too, just only from the blob, which is why we say it melts? Are there just fewer weak bonds in Rayon, so that the blob is smaller?
Actually there are two different types of nylon, [nylon 6], and [nylon 6,6].
[Nylon 6,6] is the type with "alternating bonds". It's mainly used in more industrial applications to produce solid plastics that are harder. [Nylon 6] is the type more often used in clothes. It has the same sort of "repeating" structure you'd find in a natural polymer.
Again, these two types of nylon are extremely similar to each other, it's just [Nylon 6,6] is slightly better for one type of application than another.
From a chemical standpoint, the real wonder is that the cellulose in cotton does not melt. That probably has much to do with the fact the basic sugar building blocks they are built from contain an aldehyde group and a consecutive alcohol chain. That allows the molecule to easily dehydrate (lose molecules of water) before it combusts, and also the units start becoming linked together. This makes it more solid and prevents melting. You can read about caramelization chemistry for more information.
The fact that it is a carbohydrate in overall molecular formula also helps. After the water is driven off, you are basically almost left with carbon. (However, polyester has a "carbohydrate" formula too and does not do this, it still melts)
Nylon contains 6-unit long hydrocarbon chains in its structure, like wax or polyethylene does, and these of course melt. If these were much shorter, it might behave more like wool when it burns.
A
Post a Comment